Abstract
Introduction. Gaucher disease type 1 (GD, OMIM #230800) is the most common inherited lysosomal storage disorder resulting from lysosomal glucocerebrosidase (GBA, acid-β-glucosidase) deficiency and subsequent accumulation of glucosylceramide in macrophages. This leads to anemia, thrombocytopenia and coagulopathy, visceral (hepatosplenomegaly, lungs) and skeletal manifestations (deformities, fractures, avascular osteonecrosis). Anemia is attributed to bone marrow infiltration by Gaucher cells, to inflammation and to the presence of splenomegaly; however the underlying pathophysiological mechanism hasn't been fully elucidated.
Aim. The aim of this study is to evaluate the in vitro erythroid status at baseline (T0) and the response to Enzyme Replacement Therapy (ERT) after 6 months (T1) and 1 year (T2). We evaluated the expansion and differentiation in erythroid cultures of CD34+ cells obtained from peripheral blood of GD type 1 patients at diagnosis before starting ERT (T0), at 6 months (T1) and at 1 year (T2) of ERT.
Methods. CD34+ cells were obtained by immunomagnetic separation from peripheral blood mononuclear cells of GD patients (n=3) and controls (n=5). Isolated CD34+ were evaluated by in vitro clonogenic capacity assays using the methylcellulose semisolid culture medium after 14-16 days of culture. Hematopoietic colonies (burst-forming unit-erythroid (BFU-E) and colony-forming unit-macrophage (CFU-M)) were scored with an inverted microscope. CD34+ cells were expanded in StemSpan medium, 1% StemSpan CC100 cytokine cocktail, 2 U/ml Erythropoietin (EPO) and 1 μM dexamethasone. Differentiation was induced in StemSpan medium with 10 ng/ml SCF and 10 U/ml EPO. Proliferation and surface marker expression (CD34, CD45, Glycophorine A, CD71) were examined by flow cytometry. Cell morphology was analyzed on cytocentrifuged smears stained with May-Grunwald-Giemsa.
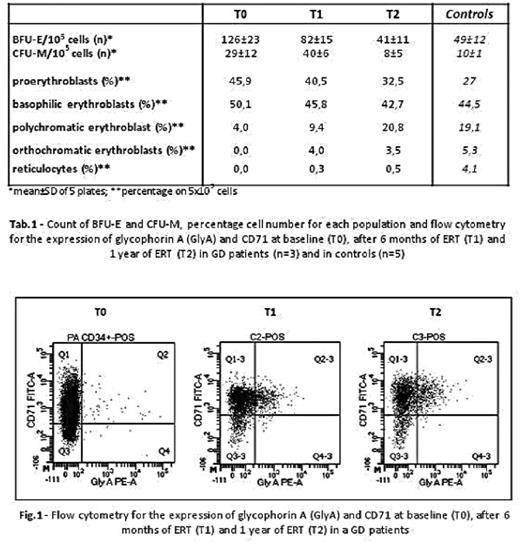
Results. At T0 we observed a higher number of BFU-E in GD samples (126±23 BFU-E/105 cells) compared to controls (49±12 BFU-E/105 cells) (Tab.1). Similarly, higher macrophage CFU-M were observed in GD samples (29±12 CFU-M/105 cells) compared to controls (10±1 CFU-M/105 cells) (Tab.1). The BFU-E and CFU-M morphology showed no difference between GD patients and controls. The increased proliferation of erythroid precursors of GD patients was associated to impaired ability to differentiate in liquid cultures after 14 days (45,9% proerythroblasts and only 4% polichromatophilic erythroblasts) compared to controls (27% and 19%, respectively) (Tab.1). Flow cytometry confirmed an impaired differentiation of GD erythroblasts compared to controls at day 14 of the erythroid liquid cultures, as shown by high percentage of CD71high/GlyAlow early erythroid precursor cells and low percentage of CD71high/GlyAhigh mature erythroblasts (GD: 73,9 and 4,9%, respectively; controls: 67,2 and 22.4%, respectively) (Fig.1). In samples from GD patients, after 1-year treatment with ERT (T2), we observed a reduction in the number of BFU-E compared to T0, reaching values similar to controls (GD T0: 126±23 BFU-E/105 cells, T2: 41±11 BFU-E/105 cells; controls: 49±12 BFU-E/105 cells) and an increased number of mature erythroid cells generated in GD liquid cultures (Tab. 1). Also CFU-M were reduced at T2 compared to T0 (GD T0: 29±12 CFU-M/105 cells, T2: 8±5 CFU-M/105 cells; controls: 10±1 CFU-M/105 cells) (Tab.1). An increased percentage of more differentiated erythroid cells at T2 compared to T0 was confirmed by flow cytometry in cultures from GD patients, as shown by low percentage of the CD71high/GlyAlow early erythroid precursor cells and high percentage of CD71high/GlyAhigh mature erythroblasts on day 14 (GD: 44,7 and 45,6%, respectively; controls: 67,2 and 22.4%, respectively) (Fig.1).
Conclusions. These preliminary data suggest that anemia in GD might partly be related to a delay in erythroid differentiation. ERT treatment seems to correct the dyserythropoiesis by improving erythroid differentiation. Since glucosylceramide accumulates in macrophages, that are key elements in the erythroid niche, impaired erythroid differentiation might be related to the pathological micro-environment, rather than intrinsic hematopoietic stem cell defect. More extensive studies are needed to better understand the pathophysiology of anemia and pancytopenia of this rare disease.This study was partially supported by Sanofi Genzyme.
Cappellini: Celgene: Honoraria; Novartis: Speakers Bureau; Vifor: Honoraria; Sanofi-Genzyme: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal